pipeline
Alzheimer’s disease
| Optimization | Pre-clinical | ADMET/CMC | Ph Ⅰ | Ph Ⅱ |
|---|---|---|---|---|
|
PO
|
||||
Shaperon also develops an oral formulation of HY209 in the treatment of neuroinflammatory diseases. The IND for phase 1 study of Alzheimer’s disease was approved by Korea FDA.
While directly targeting amyloid-β or abnormal tau proteins to ameliorate the damages inflicted on neuronal cells is the mainstream of current drug development efforts in the field of Alzheimer’s disease, Shaperon’s neuroinflammatory approach aims at microglial cells in which phagocytosis activities are exhausted and inflammation reactions are uncontrolled.
In 5xFAD mouse model, HY209 treatment showed the increase of GPCR19 expression in microglial cells which had been down-regulated by the induction of Alzheimer's disease while reducing inflammatory P2X7 expression. HY209 significantly suppresses the inflammation signaling networks in the brain, leading to the inhibition of neuroinflammatory cytokines such as IL-1β, IL-18, TNF-α, etc.
As a result, the reduction of amyloid-β plaques and microgliosis was observed while the anti-inflammatory CD47 expression was increased and cognitive functions of 5xFAD mice was improved on 10-week treatment of HY209.
IND-enabling oral formulation for Alzheimer's disease will enter phase 1 study soon with our partner, Kukjeon pharmaceutical.
-
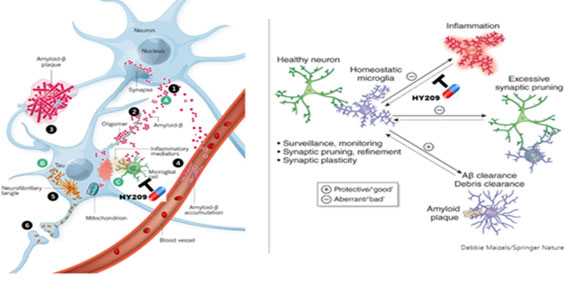
Nature, 2018, 559 (S2), Nature 2018, 559 (S16)
-
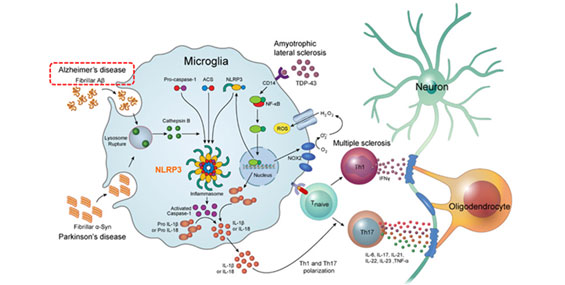
Park et al., The Open Neurology Journal, 2019, 13: 55-62